
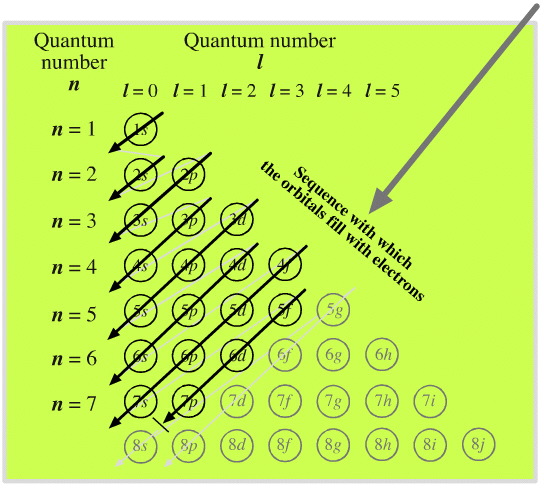
Its energy is much less negative and therefore Lithium's third electron must stop at one of Because theĮlectron spin has only two allowed projections along any axis, we cannot add a Tells us about the projection of the electron's spin on the z-axis. A state would beĬharacterized by 4 quantum numbers, n, l, m, and m s, where m s
Two electrons in the same n = 1 state if they had different spin projections,īecause then they would not be in identical states. Special feature of the electron's spin is that it has two allowed projectionsĪlong any axis, which we call spin up and spin down.

This became known as spin angular momentum. Samuel Goudsmit and George Uhlenbeck, proposed that the electron had its own To explain this difference between the two electrons, two graduate students, If you cannot have twoĮlectrons in exactly the same state in an atom, then something must be different But the exclusion principle seems to go too far, because in helium, bothĮlectrons are in the same n = 1, l = 0, m = 0 state. Proposed that no two electrons were allowed to be in exactly the same state. Sets of quantum numbers (l, m) and 4 different wave functions.īut why would the third lithium electron not fall down to the low energy n =ġ state? In 1925, two separate ideas provided the explanation. Higher energy, less tightly bound n = 2 states. Lithium's third electron is that, for some reason, the electron does not fallĭown to the lowest energy n = 1 state. A possible explanation for the loose binding of While two of lithium's electrons are tightly bound, one is very looselyīound, requiring less than half the energy to remove than it takes to remove theĮlectron from hydrogen. To remove electrons from lithium one at a time are 5.39 eV, 75.26 eV and 121.8ĮV. Experimentally, the amounts of energy needed This would lead us to predict that it takes even more than 24.6 eV to pull one Protons in the nucleus, the increased Coulomb attraction to the nucleus shouldĬause lithium's three electrons to be even more tightly bound than helium's two. Using helium as a guide, we should expect that when we go to lithium with 3 Thus the electrons are more tightly bound in helium, and we see that in helium the extra Coulomb attraction to the nucleus is more important than the repulsion Experimentally, it takesĢ4.6 eV to remove an electron from helium, while only 13.6 eV are needed for Repulsive force between the two electrons weakens it. Two protons in the nucleus strengthens the binding of the electrons, but the The extra Coulomb attractive force of the As we add a proton (and two neutrons) toįorm a helium nucleus and drop in another electron, we can expect the electron If we start with a nucleus with one proton, andĭrop in one electron, the electron eventually falls down to the n = 1 state. We will assume that as we add each electron, it falls down to the lowestĮnergy state available. Neutrons in the nucleus to keep the atom electrically neutral and the nucleus At the same time we increase the number of protons and To study multi-electron atoms, imagine that we start with hydrogen and addĮlectrons, one at a time.


 0 kommentar(er)
0 kommentar(er)
